On Tuesday, Feb. 4, visiting professor Derek A.T. Cummings at the Department of Epidemiology at the Bloomberg School of Public Health gave a talk entitled “Challenges in Infectious Disease Dynamics: highlighting work at Johns Hopkins Infectious Disease Dynamics” for the Institute for Computational Medicine.
Cummings is part of the Infectious Disease Dynamics group (IDD) at the School of Public Health — a group dedicated to combining the math and computational skills needed on a global scale to model transmission of diseases that affect millions of people globally. Some of the areas that the IDD focuses on include mathematical, statistical and computational models; analysis of sequencing data on pathogens; and data integration within empirical studies.
“We're grounded in data, and we work on a number of different pathogens and a number of different locations,” Cummings said.
Cummings specializes in studying the interaction between infection and immunity throughout an individual's lifetime, including how repeated influenza infections shape influence immune responses and future infection risk. He is also interested in developing methods to analyze sparse data by integrating mechanistic models of pathogen transmission and dynamics. Additionally, he uses genomic data to understand transmission patterns, selection processes and pathogen evolution.
Cummings started by explaining the mathematics involved in modeling disease dynamics. Much of the work is done using systems of differential equations, such as the susceptible-exposed-infected-recovered model. His research also encompasses stochastic processes, immunodynamics of infectious diseases and dynamic systems statistical inference.
The IDD and many other infectious disease groups face many challenges in modeling disease transmission. One of the most prominent challenges they face is describing transient phenomena. There are factors that need to be incorporated into the data that can make systems incredibly complicated and computationally heavy. Incorporating viral genetic data into models adds another layer of complexity. For example, phylogenetic trees (which show the evolutionary relationships between viruses) can help track the spread of disease, but analyzing these trees is computationally expensive.
“One major problem we face is that we're often looking at transient phenomena. It's something that's difficult for us. All of a sudden, you incorporate the state of the system before you start seeing the data, and it really expands the possible space of what could be happening,” he explained. “We often don't know where we are in that transient phenomena, and then the system is changing.”
Some of the work with far-reaching applications that Cummings assisted the IDD with was making a model to be used in national response efforts during the COVID-19 pandemic and characterizing people’s immunity to influenza for the same purpose across the world.
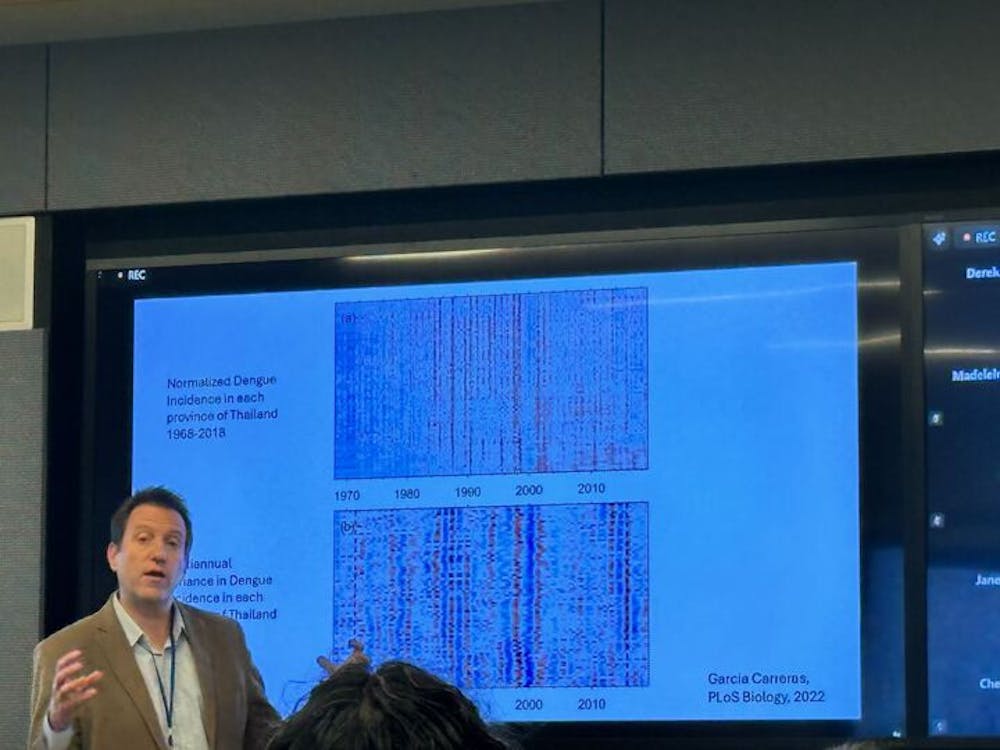
To offer insight on what the group has accomplished in terms of modeling transient disease dynamics, Cummings provided case examples. Dengue — a highly infectious disease in Southeast Asia — has always been of interest to him and his colleagues due to the cyclic nature of outbreaks and the associated public health impact.
“2024 had the highest number of dengue cases ever reported globally, largely due to a massive epidemic in the Americas,” he said. “Hospitals ended up converting entire wards exclusively for dengue patients, or converting sports stadiums as alternative care facilities. This boom-and-bust cycle of dengue has a significant public health impact.”
With the impact of outbreaks on public health, Cummings set out to model the factors impacting dengue outbreaks. Using data from the Ministry of Public Health in Thailand, Cummings made several observations about the outbreaks that could be modeled using a differential equation.
Higher temperatures shorten the incubation time, leading to more efficient transmission. Immunity also plays a role; after a large outbreak, the pool of susceptible individuals is depleted, making it harder for the virus to spread even if temperatures are favorable. In Thailand, dengue outbreaks across different provinces oscillate between periods of synchrony (where all regions peak at the same time) and asynchrony (where peaks are staggered). This synchrony is driven by a combination of temperature fluctuations and immune dynamics.
“Our conclusions from building these models is that the patterns of synchrony really demonstrate the interplay of immunity and temperature, and your immunity is working,” he said.
In the spread of dengue, broad movements of people also play a role. Using viral genetic data, researchers found that dengue viruses are highly localized within small geographic areas during a single season, but they become homogenized across the country over longer periods of time. As such, having data on where people move and potential pathways of transmission, which can be accomplished with integration of phone call data or viral genetic data, can be powerful tools for predicting disease dynamics.
“Because the virus is a tracker, you can sequence it, and it has a mutational process that's making it different, which is basically associated with time and separation by generations,” he added. “If you have a bunch of sequences, you kind of have a tool to understand the interactions of pathogens.”
Applying this method to influenza, Cummings is working on an influenza antigenic map, which estimates functional immunity to viruses across different mutations spatially.
“This antigenic map says that, if I were infected with this, then my level of protection to this other virus, which is basically one square away, is half as functional as if I were exposed to the very same virus that I was exposed to... the highest risk is when they're at sort of mid-distances,” Cummings said.
Using an antigenic map to show how viruses genetically have moved further apart over time, Cummings suggested that dengue evolves to evade immunity, similar to influenza. However, older data reveals a more complex pattern: Viruses initially started of genetically distinct, moved closer together by the early 1990s and then diverged again. This indicates a pull and push dynamic — where viruses balance evading immunity with exploiting pre-existing immune responses to enhance genetic transmission.
While the growing availability of large datasets help address some issues, they also introduce new complexities, such as managing and integrating high-dimensional data. Multiscale simulation and inference also remain difficult, particularly when integrating a wide variety of processes ranging from pathogen genetic to contact networks. Describing transient phenomena, like invasion dynamics and population responses, is especially challenging due to the rapid and unpredictable nature of these events.
Additionally, studying evolutionary time scales is complicated by the transient nature of pathogen dynamics, making it difficult to draw long-term conclusions from short-term observations. Despite these challenges, Cummings and his colleagues are determined to improve the computational efforts on these problems to reduce burden on public healthcare systems.
“On evolutionary time scales, that's still a pretty small time slice to study these phenomena, and it's inherently transient, so dealing with transients in sort of dynamical systems and matching these systems is something that we're definitely finding difficult,” he concluded.