On Tuesday, Nov. 5, as part of the Johns Hopkins Medicine’s Department of Neurology and Neurosurgery Neuroimmunology Seminar Series, Dr. Amber Salter delivered an overview of her ongoing work concerning comorbidities in multiple sclerosis (MS) disease, titled “Examining Associations of Comorbidities in MS Disease-Modifying Therapy Clinical Trial.” Salter is an associate professor of Biostatistics at UT Southwestern Medical Center.
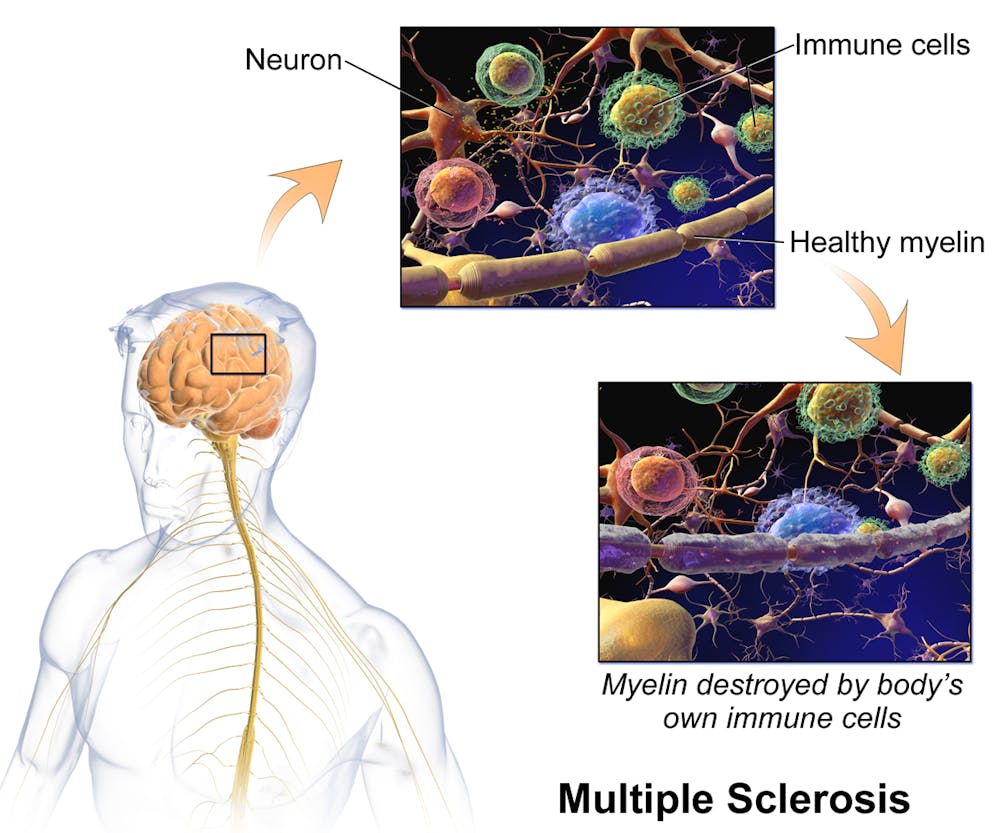
MS is a chronic autoimmune disease of the central nervous system where the in which the immune system mistakenly attacks the body’s own tissues. Particularly, it targets the myelin sheath — a lipid-rich coating on the neuron’s axon — impairing the ability of nerves to conduct electrical signals, leading to chronic nerve damage.
Comorbidity refers to the presence of additional health conditions alongside a primary disease being studied, contributing to the overall health burden on a patient. Comorbidity is common in MS patients, with the most frequent being depression, anxiety, hypertension, hyperlipidemia and chronic lung disease.
In Salter’s lab, she and her colleagues are particularly interested in the influence of MS comorbidities on the clinical outcome and prognosis of MS. She explained that there are multiple benefits in studying comorbidity, including improved prognostication, more personalized treatment plans, better risk stratification, and, ultimately, the development of management strategies for comorbidities within MS to improve clinical outcomes for patients.
There are multiple barriers to completing her lab’s goals, however. As Salter explained, gaining access to diversified and representative MS clinical trial data has been relatively tedious, especially with phase 3 data (comparing how well the new treatment works to old treatments). One particularly concerning issue was the limited availability of comorbidity status recorded, leading to the underrepresentation of individuals with comorbidities in clinical trials. As such, treatment outcomes can be heterogenized or undeserving MS patients who need comorbidity management along with MS treatment.
Salter also elaborated on multiple limitations of past literature on MS comorbidities, with problems in study design and measurement methodology. She explained that preceding studies have utilized observational methods rather than clinical data, which doesn’t fully elucidate the scope of the issue. As such, data on comorbidities and MS clinical outcomes remains mixed.
Seeing this, Salter and her team sourced data from 17 trials, which totaled more than 17,000 participants, and answered the knowledge gap based on two objectives.
“One, to estimate the prevalence of comorbidities in phase 3 clinical trials on MS disease-modifying therapies and two, to evaluate the association between comorbidity and disease activity on clinical and imaging outcomes in Phase III clinical trials of MS disease-modifying therapies,” she said.
For the first objective, Salter’s group analyzed individual data from the multiple sponsors for phase 3 trial data and categorized comorbidities using medical history data. Several categories were identified, with overarching labels concerning autoimmune, cardiometabolic, migraine, lung, psychiatric and skin. They looked at both individual comorbidities and the sum of the comorbidities as well. Overall, her team found that 25% of MS patients across the trials had one comorbidity, 11% had two comorbidities and 6% had at least three. Comorbidities prevalence was also divergent across demographics (sex, age, race, etc.), which, according to Salter’s group, highlights the need for more inclusive clinical trial enrollment.
Salter stressed that when pooled across trials, over half of the participants had at least one comorbidity, further emphasizing the pressing nature of considering comorbidity in MS treatments and improving the prognosis for patients.
“It will be important to further understand the influence of comorbidity on outcomes in clinical trials,” Salter explained.
For the second objective, Salter was interested in whether MS comorbidities swayed the clinical outcomes, which were modeled by observing evidence of disease activity beginning and end points, worsened disability, and contributed to relapse and/or new brain lesions. Using the same data set as before, Salter’s group reported a 61% pooled proportion of evidence of disease activity, simultaneously highlighting that having over three comorbid illnesses as an MS patient increases disease activity and risk. Having just one comorbidity could increase patients’ risk of relapses in MS, and having over three comorbidities also places patients at disproportionate risk of disability worsening,
When investigating safety outcomes for individuals with comorbid MS, Salter reported that increased comorbidities correlated with increased risks of adverse health events associated with MS, such as infections, treatment-emergent autoimmune diseases and cancer.
“Comorbidity burden and comorbidity are associated with worsening clinical outcomes,” Salter emphasized. “In the broader MS community where comorbidity burden may be higher, the prevention and management of comorbidity is a pressing clinical concern.”
Salter aspires to address treatment effect modification and response heterogeneity in the near future. She seems excited about the possibility of clinical trials to evaluate whether treating comorbidities improves outcomes and explore possible tailored treatments for MS patients with comorbid diseases.