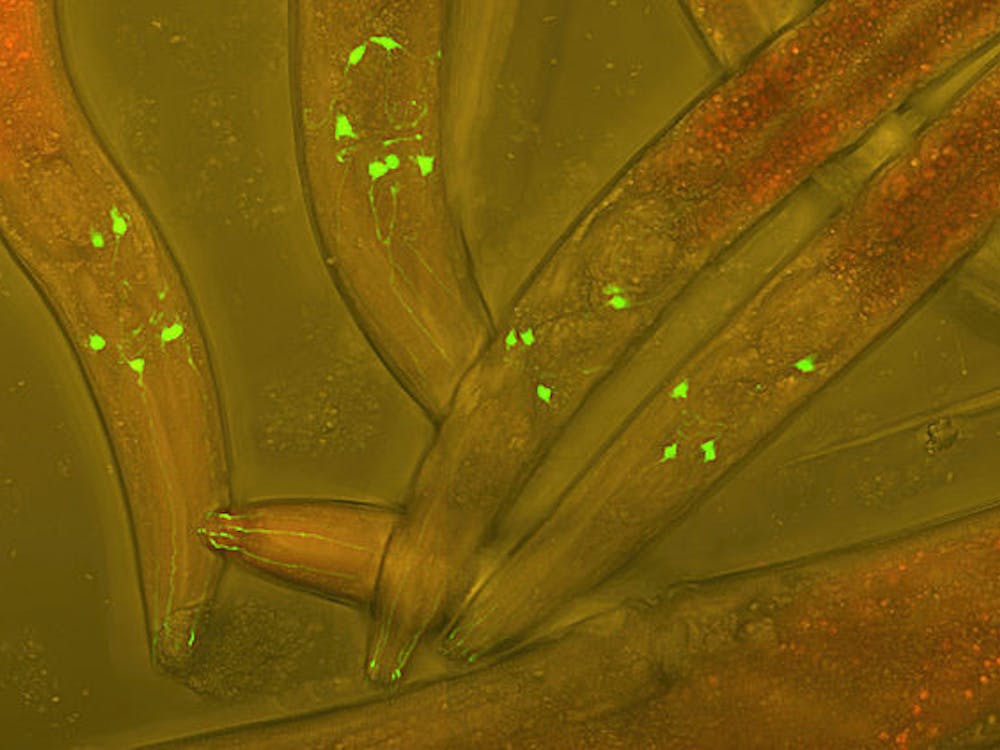
A team of Hopkins researchers identified the role of Histone H3 in regulating cell plasticity in worm embryos. Their results were recently published in Sciences Advances.
Histones are proteins that give structural support to chromosomes by allowing DNA to wrap around them and stay in a more condensed shape. H3 is the histone of interest in the study, and the researchers studied functions of H3 histones in worm embryo cells.
Ryan Gleason, a postdoctoral fellow at the Department of Biology run by Xin Chen, is the lead author of this publication. In an interview with The News-Letter, he explained why his team chose to study worms, namely Caenorhabditis elegans.
“Worms, in particular, are like a factory of embryos. They have germlines that are easy to identify under scopes and produce embryos at a relatively fast speed that is optimal for investigation purposes,” he said.
According to him, there are other canonical models that the lab uses to study cell differentiation, such as yeast, but it might not be ideal to study germline-to-embryo transition.
The researchers used CRISPR-Cas9-mediated gene editing, which borrows its inspiration from a bacterial immune defense system, because it allowed them to modify individual genes and examine the roles of histone genes. CRISPR-Cas9-mediated gene editing creates a “guide” RNA that is complementary to the target DNA and binds to the Cas9 enzyme. After introducing the “guide” RNA into a cell, it recognizes the intended DNA, which is where the Cas9 enzyme cuts. Traditional methods such as RNA sequencing would not have given access to editing individual genes like CRISPR-Cas9.
The researchers found that increasing the H3 level was associated with decreased cellular plasticity of pluripotent cells in worm embryos. Pluripotent cells are cells before differentiation that have the potential to become specialized cells, such as neurons and muscle cells. When the H3 level was reduced, the duration of pluripotency for cells in worm embryos was extended. Based on this, the researchers concluded that modifying the H3 level can regulate normal embryogenesis pathways and make the embryo cells adopt an alternative cell fate.
Gleason noted the importance of the differential regulation in the transition from pluripotency toward specialized cells.
“[H3] was kept low early during pluripotency. Then upon differentiation, there is a developmental or epigenetic switch that happens to incorporate all 15 [genes],” he said.
Histone H3 was the first oncohistone found to have frequent mutations in various types of cancers in humans, such as osteosarcoma and acute myeloid leukemia.
Gleason examined the implications of the study’s findings on understanding Histone H3’s function.
“It is applicable to the mechanistic level as far as what the histones are doing, or potentially doing when they're mutated. And depending on the type of cancer you have, maybe it one day could be possible to CRISPR edit back to a normal state,” he said.
In an email with The News-Letter, Chen, who is a co-author of the study, reflected on the overall research process and the challenges encountered by the team.
“You definitely need to be resilient and stay optimistic when things do not work out. It is also important to learn from failed experiments to avoid making the same mistakes,“ she said. “Knowing how to not get frustrated and even enjoying this process is absolutely needed when doing research.”