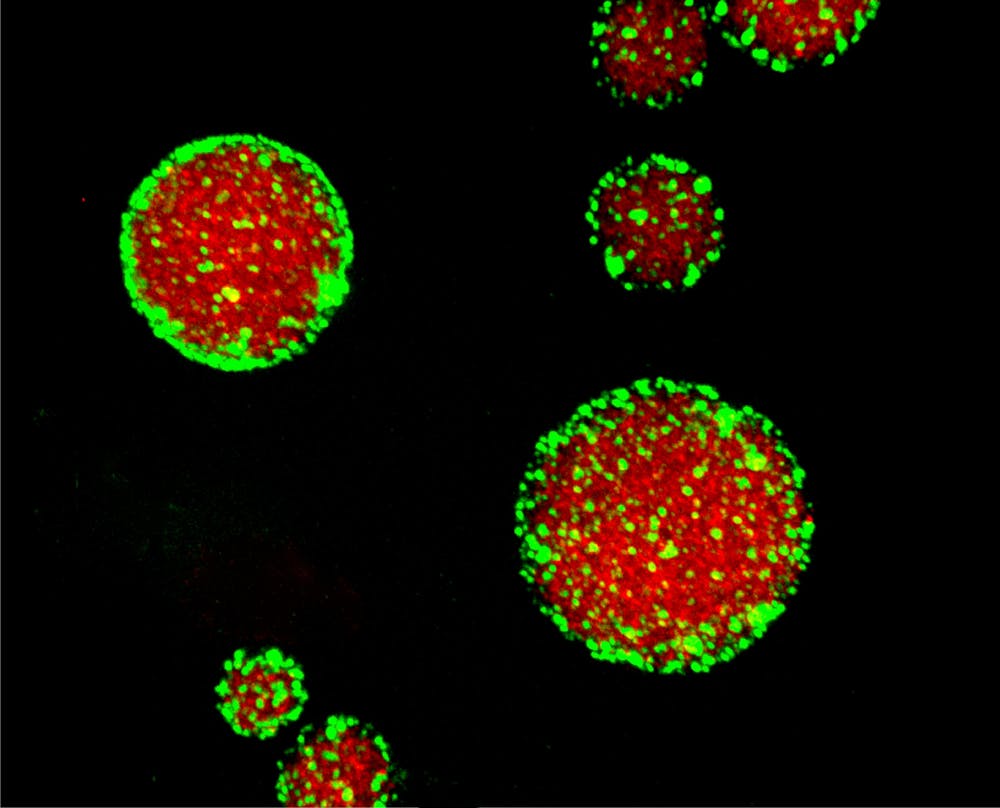
Geraldine Seydoux, the Huntington Sheldon professor in medical discovery at the School of Medicine, and her lab discovered protein clusters that adsorb to the biomolecular condensates in the gelatinous interior of a cell. These protein clusters, MEG-3 and MEG-4, mimic the function of lipid-bilayer membranes that encapsulate organelles.
Seydoux elaborated on the research in an interview with The News-Letter.
“The traditional view of organelle is an organelle that has lipid bilayers,” Seydoux said. “Then about 10 years ago, scientists saw [biomolecular condensates] that did not have any membrane. The idea is that these condensates are just like oil droplets that do not mix with water.”
P granules, condensates in the Caenorhabditis elegans germline, are the main interest of Seydoux’s investigation. They are membrane-free, highly dynamic phase-separated liquid droplets that play a crucial role in the germ-cell fate of C. elegans.
It was previously believed that membrane-free P granules were only a combination of RNA and proteins. However, Seydoux and her team, including two post-doctorates and a bioengineer, found something unusual.
“These P granules are highly dynamic organelles that can dissolve on one side of the membrane and reform on the other side,” she said. “These P granules are different from [membrane-bound] organelles because it takes longer for them to divide and grow.”
The uncommon patterns of behaviors observed in P granules led Seydoux and her team to suspect that these are not typical organelles.
Despite the observed unusual patterns, there was still no concrete evidence for Seydoux to conclude that these P granules were more than biomolecular condensates merely floating in the cytoplasm. The actual breakthrough of this investigation came when they looked further into the phase-separation condensation of the protein.
“Phase-separational condensation is the phenomenon in which proteins [that have an affinity with each other] in the aqueous buffer will phase separate and will make concentrated protein condensate,” she explained. “However, in the cells we do not see [phase-separation condensation]. These droplets stay nicely separated from one another.”
To tackle this problem, Seydoux and her team used genetic methods to investigate the surface of the P granules. It was later found out that there were protein clusters that could adsorb to the surface of P granules. These protein clusters, serving the function of a Pickering agent, a kind of emulsion stabilized only by solid particles locating at oil–water interface. Pickering agents are used as emulsions in the food, cosmetics, and pharmaceutical industries.
In P granules, the protein clusters sit on the interface between the interior and the exterior of P granules, allow P granules to reach thermodynamic equilibrium with their surroundings and keep them separated from each other.
“That is essentially the functional equivalent of the membrane. The old definition of membrane is lipid-bilayer. Now there is a totally different understanding of lipid-bilayer,” she said. “These protein clusters prevent droplets from fusing with each other.”
The protein clusters — MEG-3 and MEG-4 — provide more than the function of a Pickering agent. They are able to recruit a kinase (MBK2) to phosphorylate the interior of P granules and allow the diffusion of molecules. This discovery provides further evidentiary support to the belief that the protein cluster reminisces the function of a membrane.
“The Pickering agent, in this case, is able to increase the dynamics of condensates,” she said. “This is just like a membrane [because] membranes don’t just encapsulate; they have channels that allow molecules in and out.”
The protein clusters are also responsible for localization of the biomolecular condensates. Seydoux elaborated on this phenomenon by investigating the function of MEG-3 and MEG-4 in driving the asymmetric growth of the P granules during the polarization of the C. elegans zygote.
“These little granules are bringing all the things needed to make a germline,” she said. “MEG proteins regulate the dynamics of granules by segregating [different] granules to [corresponding] ends of the embryo.”
The next goal of this project is to understand the specific function of the P granules in the germline fate of embryos. However, the detailed mechanism has not been completely understood yet. Seydoux and her team are currently using proteomic techniques to identify specific molecules inside the condensates and their relevant function in the germline fate.
“What we think right now is the RNA and proteins need to go to the germline to specify the cell fate. What that means in practice we are not completely sure,” she told The News-Letter. “We first catalog [molecules] inside and then understand how these molecules allow embryonic cells to become gametes or oocytes, [et cetera].”
Seydoux and the team have already filed patents on the use of MEG-3 as a tool for developing Pickering emulsions. Pending further research, MEG-3 could eventually provide a renewable resource for developing Pickering emulsions in the food and chemical industry.