The prefix “epi” typically connotes being above or at the surface level of something. However, research at the intersection of epigenetics and epidemiology probes our understanding of the interaction between genes and environmental factors to the deepest levels.
In the current crisis of the coronavirus (COVID-19), some scientists are investigating how the family of coronaviruses affects the regulation of the human genome.
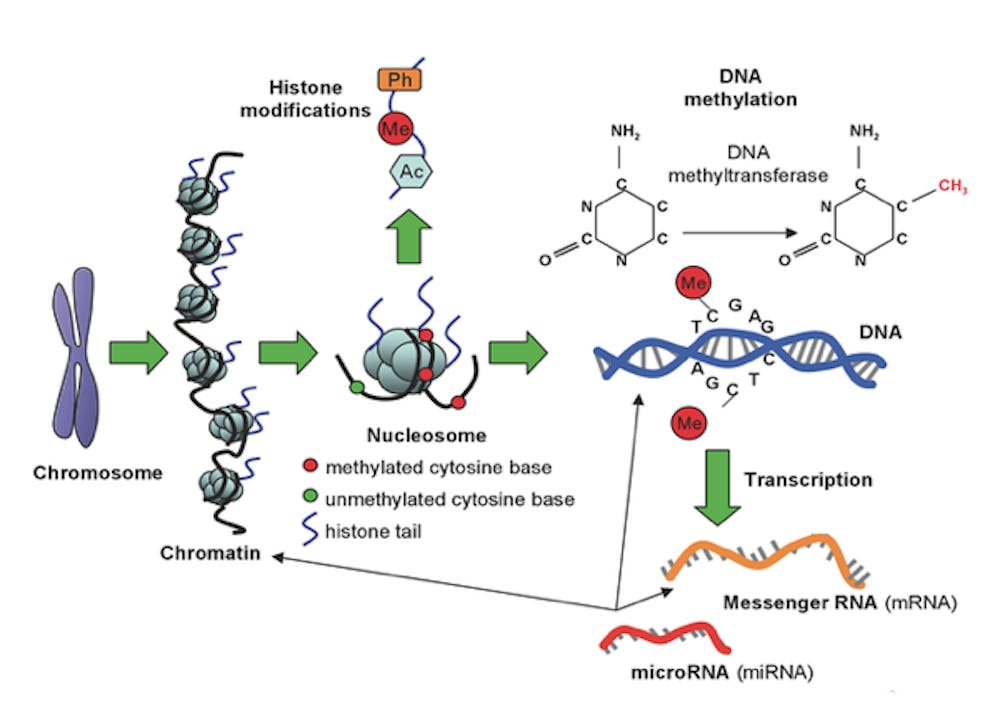
Current research in epigenetics focuses on the effects of molecules and processes on DNA, rather than emphasizing the role of the genetic code itself. Major areas of focus include chromatin remodeling, histone methylation and many other processes that affect how transcription of DNA is initiated as well as how DNA is packaged in a cell.
The complex interactions of coronaviruses and epigenetic processes of a human cell have been researched for some time, especially following the completion of the Human Genome Project and the outbreak of past coronaviruses such as SARS.
A 2017 review paper in the journal Pathogens titled “Epigenetic Landscape during Coronavirus Infection” details the various ways in which coronaviruses affect the transcription initiation machinery of a cell and how those changes alter the regulation of DNA replication and transcription. These processes are critical for the virus since it relies on the host cell to replicate its genetic material and continue to proliferate.
Understanding how coronaviruses force the host cell to transcribe viral genetic material can help lead to vaccines and therapeutics that can prevent the proliferation of the virus by targeting its replicating mechanisms. For example, the Icahn School of Medicine at Mount Sinai and other medical schools are in the midst of developing an epigenetics test that will allow for early detection of the novel coronavirus that leads to COVID-19.
Mark Daly and Andrea Ganna of the University of Helsinki’s Institute for Molecular Medicine Finland recently developed the COVID-19 Host Genetics Initiative which brings together geneticists to find the genetic determinants of COVID-19 susceptibility.
Philip Murphy, an immunologist at the National Institute of Allergy and Infectious Diseases suspects that variations in the ACE2 gene can make some people more susceptible to COVID-19 than others. UK Biobank, FinnGen, deCODE Genetics and the Personal Genome Project are among the collaborators collecting genome data from consenting COVID-19 patients.
However, according to Dr. Andrew Feinberg, environmental factors may have a greater bearing on disease onset than genetics. Feinberg, a Bloomberg Distinguished Professor in the Hopkins School of Medicine, researches precision dietetics, cancer, neuropsychiatric disease and infectious disease adaptation. In an online lecture earlier this month, he said that only five to 30 percent of diseases are currently understood through the whole sequence of the human genome.
Feinberg described the genome as not static, and spoke to the connection between epidemiology and epigenetics.
“The genome is adapted to its environment,“ he said. “It’s a way of bridging the gap between clinical care and traditional epidemiological approaches to environmental studies.”
Epidemiologists study disease prevalence and incidence in populations, particularly the effects of environmental factors on human health and bodily function. These include the effects of smoking and various diets on the body.
An example that Feinberg discussed was the link between increased DNA methylation and high consumption of methionine, an important amino acid used to make proteins in the body.
Giving methionine in varying quantities prenatally to mice has identifiable effects of phenotypic variability in the offspring. Feinberg stated that this recent research showed how different diets such as the Western or Mediterranean diet can lead to different amounts of methylation in specific genes.
These efforts focus on investigating how specific environmental factors affect the regulation of genes and how these changes to regulatory pathways and genetic diversity may be passed onto future generations.
Researchers at the University of Copenhagen determined that diabetes, obesity, hypertension, cardiovascular disease and lung disease are all risk factors for COVID-19 infection. A study found that, compared to patients in non-intensive care, twice the amount of patients in intensive care had diabetes.
According to Feinberg, various risk factors for specific diseases can be quantified by analyzing DNA methylation of specific genes and other epigenetic markers.
“Future directions for such research include looking at epigenetic adaptations in regards to predictive markers, new targets and the associated mechanisms,” he said.