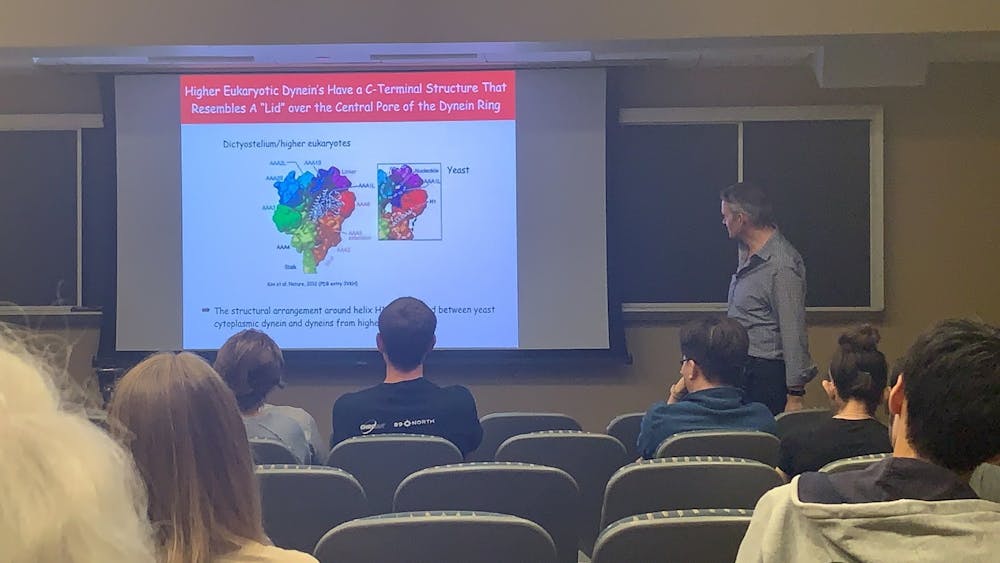
On Nov. 7, the Department of Biology Seminar Series hosted Arne Gennerich of the Albert Einstein College of Medicine. Gennerich utilizes single-molecule technology to probe the function and mechanisms of dynein, a motor protein in our cells.
Single-molecule technology allows researchers to hone in on one molecule, rather than observing a population average. By employing it to observe dynein, Gennerich researches how the motor protein can carry a load to different cellular destinations while “walking” across microtubules, which web across the cell like railways.
Dynein contains two polypeptides that partly form rings, called “heads.” Extending from these heads are two stalks, like legs. As if wearing suction cups, the leading stalk cyclically binds and detaches from the microtubule, while its partner stalk attaches to the cargo.
Gennerich described the importance of dynein in diverse processes.
“If you were to hurt your toe, ask yourself, how many days [would it take to start] to heal? It turns out if transport would rely on effusion, it would take 107 years. But it takes only a few days for molecular motors that [are] able to move along the nerve towards the toe.”
Even neurons that migrate to the surface of the brain, chromosomes that are pulled into daughter cells during cell division, and activated T cell receptors that concentrate in the center of cell are driven by cytoplasmic dynein.
If mutations pop up in the gene regulating cytoplasmic dynein, diseases like microcephaly can occur.
When functioning, however, dynein can take thousands of consecutive steps forward while transporting cargo.
“This means that the leading motor domain... must stay bound while the trailing motor domain...
must know that it’s in the back [in order to turn] in[to] a weak binding state,” said Gennerich. Even though both motor domains are identical, they are always out of phase.
To understand how this motion and transport is done on a molecular scale, Gennerich and his colleagues developed technology that combine single-molecule fluorescence and optical tweezers.
Single-molecule fluorescence relies on tagging the molecule of interest with fluorescent proteins and studying the emission and reabsorption of photons. This emission and reabsorption changes with time as distance changes, allowing researchers to study the internal dynamics of a molecule.
Optical tweezers use laser beams to trap and manipulate a molecule, which can be pulled like taffy to measure the amount of force necessary to disrupt the structure.
Blending these techniques, Gennerich’s lab studied how the two heads of dynein, although identical in structure of motor domains, “know” where they are relative to each other in order to keep out of phase.
Gennerich hypothesized that the rings that comprise the heads of dynein prefer to be stacked.
“[However,] based on step size and [further] analysis, we envisioned that they splayed apart,” said Gennerich.
This means that within the same molecule, the leading head feels tension tugging it backwards, while the partner head feels tension pulling it forwards.
With that in mind, Gennerich’s lab designed an experiment to probe whether the backwards tension that the leading head feels in fact increases the strength of the binding between the leading head and the microtubule it walks along. Conversely, does forward tension pulling the partner head decrease the binding strength of that partner head?
By engineering a dynein with a fluorescent protein in place of a head, Gennerich and his colleagues could measure the binding strength. They found that dynein changes its binding strength in the direction in which it is pulled. After an initial increase in strength as force applied on dynein increases, dynein becomes insensitive to any more increase in force.
In order to intramolecularly communicate, two helices within the stalk “slide.” This helix sliding must occur in order for dynein to alter its binding affinity. But some key piece was missing — what could induce sliding through tension?
Gennerich’s lab hypothesized that the buttress, a structure found below the stalk, was involved. He and his colleagues soon discovered that without stalk-buttress interactions, the stalk helices could only occupy a state of minimal binding.
“This tells [us] directly that the buttress mediates tension-induced helix sliding.” In other words, the buttress and stalk must communicate in order for the helices to regulate their binding affinity, and for dynein to be able to take thousands of consecutive steps forward along microtubules.
Riti Gupta, a seventh-year PhD candidate whose work also centers on single-molecule techniques applied to motor proteins, attended the presentation. She remarked on the precision of his methods in analyzing dynein can be applied to understand where differences between the work of different scientists arise.
“I really liked specifically what he said about how tagging the protein at a specific side can affect the results that you have as well as concentration. [By] pinpoint[ing] where those differences come from... [we can decide whether] experimental conditions need to be changed or examined more carefully.”