On Oct. 10, the Chemical and Biomolecular Engineering Seminar Series hosted Sarah Heilshorn of Stanford University. Heilshorn’s research bridges materials science — the study of how the structures of different materials affect their functions and properties — and clinical impact.
Typically materials scientists either synthesize new materials or harvest naturally derived products for medical applications.
Although researchers can direct the functions and properties of materials by defining their structures, these materials rarely biodegrade in our bodies, meaning they do not break down easily into organic matter and enter the environment harmlessly.
To avoid this, these synthetic materials must often be modified.
In contrast, natural materials interact well with our bodies, but they have been evolutionarily refined for specific purposes; in other words, they are difficult to modify.
By bridging both approaches, Heilshorn and her colleagues are like DJs of science: They take existing products, in this case natural, biodegradable amino acid sequences, and mix and match them in order to synthesize brand new polypeptides.
In fact, protein engineering has allowed Heilshorn to modify properties of materials used in the treatment of spinal cord injuries (SCIs), for which she asserts that the life expectancy for patients has largely not changed since the 1980s. Heilshorn explained why she believes this is.
“We now, within the medical community, are very good at keeping those patients [simply] stabilized and alive,” she said in her presentation. “This is because regenerative therapy, in which delivering healthy cells in place of drugs is central, has hardly progressed and few clinical trials have succeeded.”
SCIs can cause cavities to form in the spinal cord. To fill those cavities, Heilshorn’s colleagues turned to Schwann cells, whose primary function is to protect neurons in the peripheral nervous system (PNS).
They isolated Schwann cells from the PNS, expanded them and redelivered them into cavities to promote surrounding tissue to regenerate and function. This process decreased the size of cavities and prevented surrounding tissues from further degrading, but the damaged tissue had only regained “moderate” function, her colleagues found. This is because recovery of function is associated with the number of transplanted Schwann cells.
Few Schwann cells ever reach the target tissue, and fewer survive a month later.
This indicates a delivery problem. Heilshorn explained that this is where she, her colleagues, and their engineered proteins stepped in.
“Our goal initially in this project [was] to try to identify... the key bottlenecks that are responsible for this decrease in cell viability and [to] design material to overcome those challenges,” Heilshorn said.
To do this, the researchers set out to design a material that could be injected into the spinal cord and successfully ferry Schwann cells from the syringe needle to the target location. They identified three stages of the delivery process, the “bottlenecks,” that the biomaterial would have to protect its cells from.
Heilshorn said that their first concern was a constriction point that could cause cell membranes to fracture, leading to cell death.
“If you encapsulate the cells in a weak, visible gel that’s shear-thinning, the gel can form a lubricating layer near the edges of the syringe needle and the rest of the gel can slip through... which provides mechanical protection to the cell membrane,” Heilshorn said.
In other words, cells can sneak into the body as if engulfed by bubble wrap.
The second obstacle was the positive pressure the spinal cord endures.
When removing a needle from a patient, a “geyser of cells and fluids” can spray out, Heilshorn described.
The biomaterial, still encapsulating the cells, must thus stiffen when injected to counter the positive pressure.
The third hurdle was cell die-out.
Without a matrix to stick to, cells instead adhere to each other and clump within spinal cord cavities, refusing to bridge gaps between neurons.
By designing a biomaterial that offers a matrix, but not one so heavenly that cells would refuse to migrate to surrounding tissue, researchers might encourage the cells, like baby blue jays, to take flight.
And from these caveats sprang Shear-thinning Hydrogel for Injectable Encapsulation & Long-term Delivery (SHIELD, name and function very much noted).
Simply physically mixing two liquid polymers encourages them to self-assemble into a protein network that can envelop cells.
Typical clinical trials deliver Schwann cells through saline.
However, compared to injury-only controls, SCI rat models treated with SHIELD displayed a statistically significant increase in the number of successfully transplanted cells and the number of cells that survived 1 month later (the length of the clinical trial).
Functional recovery is associated with the number of cells, and indeed, these rats treated with SHIELD had improved grip strength and coordination. Additionally, not only were more Schwann cells present, but these cells were also elongated, suggesting that the cells had migrated farther into the surrounding tissue.
Heilshorn explained that he believed this raised a key question: should we continue to design materials for a specific use at a specific time, or take time as a variable?
“Perhaps we need to often also consider the life-cycle of the device as it’s being implemented by the surgeon, as it’s delivered, as the cells change their phenotype,” she said. In other words, materials should be modifiable before and after delivery.
Heilshorn then presented a second, shorter research vignette.
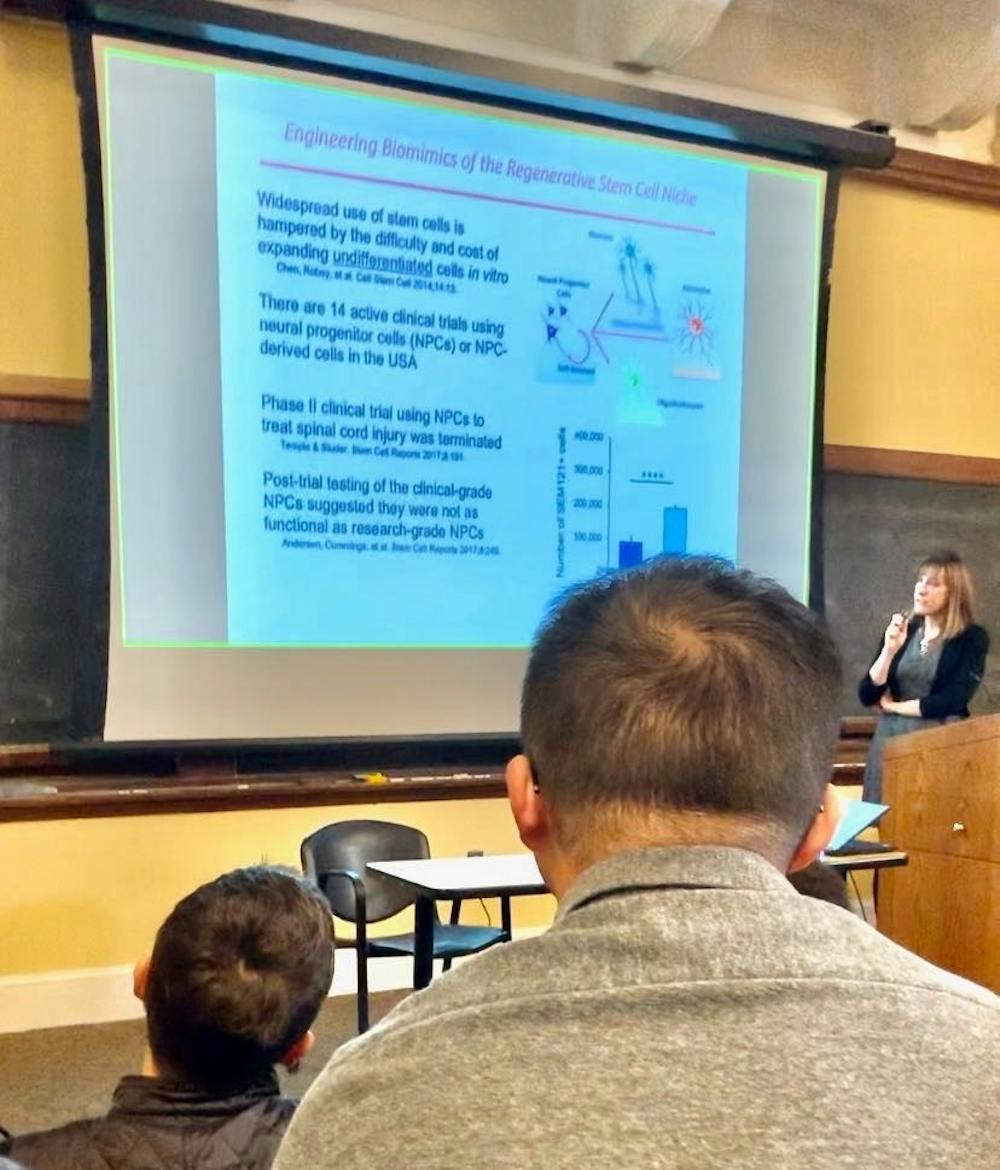
Neural progenitor cells (NPCs) are stem cells that are difficult and expensive to expand without inducing differentiation.
Although a clinical trial using NPCs was terminated, after which it was found that the NPCs used in patients were not as functional as those in research laboratories, Heilshorn noted that 14 active trials still use them.
The key to using these NPCs as therapy, however, might be modeling them in a 3-dimensional system, as opposed to a 2-dimensional culture.
This would reduce the amount of media needed and expose the cells to a “homogeneous” environment. But what would offer a successful 3D expansion system? Hydrogel.
But, as Heilshorn stated, NPCs misbehave in 3-dimensional gel systems - they lose their stemness (their ability to proliferate, display certain protein factors, and differentiate).
What type of material, then, would help NPCs maintain stemness?
Above all, the protein-engineered gel would have to be stiff and biodegradable.
This would encourage cells to adhere to the cross-links, which are tightly interwoven, and be in contact with each other. In remodeling the matrix, the number of NPCs and their regenerative function are retained.
These research vignettes, however, are not just specific to SCIs. They suggest a potential for broader impact: Heilshorn and her colleagues’ work not only bridges materials science and medical applications, but also pioneers the way we approach cell delivery.
In an interview with The News-Letter, senior Amanda Li expressed her belief that Heilshorn’s research could apply in multiple situations.
“Her work can apply to a lot of different biomaterials... so she has the potential to enhance cell delivery for cells as a drug or a therapeutic in any situation, especially because we’re now turning towards a lot of regenerative medicine,” Li said.