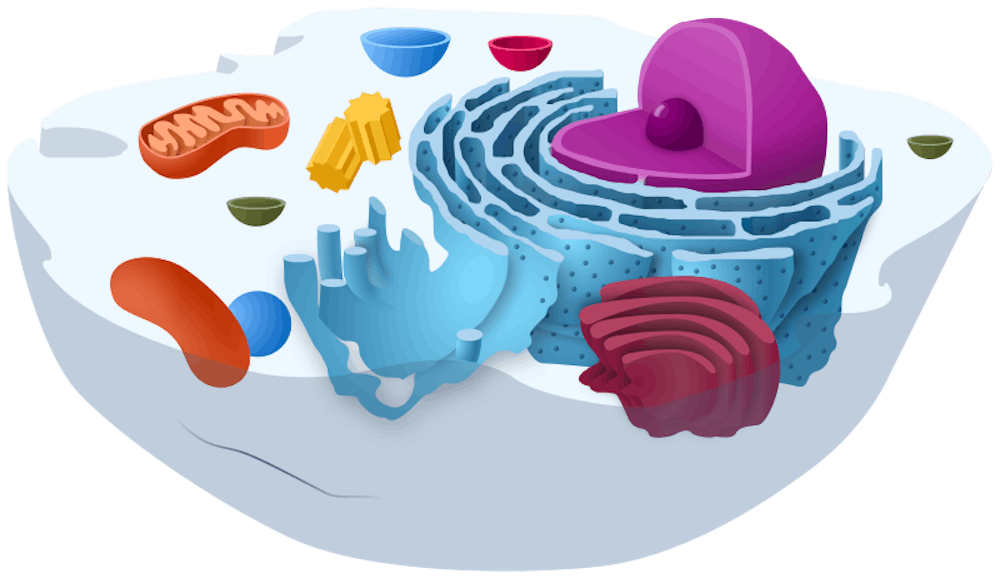
Biology is founded on a few main theories: cell theory, that all life is made up of cells and all cells on Earth come from previous cells; gene theory, that traits are passed down to offspring through genetic material; and evolutionary theory, that heritable characteristics change in populations due to natural selection.
These three ideas collectively explain why living things share similar characteristics, how new species occur and what the smallest unit of life is. Still, many questions remain to be answered about how life got here and how the insides of a cell work.
One unanswered question is how cells are able to generate and consume energy and use it for growth. While this may seem like a simple enough question — after all, the mitochondria is the powerhouse of the cell — the answer is actually quite complicated.
Cells typically have multiple energy production pathways so they can be adaptable to different environmental conditions. But a fundamental question in biology is how cells are able to organize all of these individual pathways, which may overlap with other pathways.
Adenosine triphosphate (ATP) is perhaps the most notorious “energy molecule” used by enzymes to perform work within cells.
A new study by Yu Chen and Jens Nielsen at the Technical University of Denmark attempts to shed light on the energy spurring processes in escherichia coli and baker’s yeast by shifting their metabolism from fermentation to respiration states, causing them to produce more ATP. It also proposes that energy metabolism should be modeled by breaking it down into biomass formation pathways and ATP-producing pathways.
They found that ATP can be generated in two ways within cells. The first is the “respiratory pathway,” which generates nearly 24 ATP molecules for a single glucose molecule. The second, the “fermentative pathway,” can only pump out around 11 ATP molecules for every glucose molecule input.
Both pathways exist in a natural balance that was shifted by the researchers experimentally, by varying the amount of sugar and protein available to cells.
The researchers found that cells needed more protein mass to activate the high-yield respiratory pathway than the lower-yield fermentation pathway, despite the fact that they were consuming glucose at the same rate.
If key enzymes were made to perform better, then cells would trigger a metabolic switch from fermentation to respiration, which results in more ATP and prevents the byproducts of fermentation from building up.
More ATP and fewer byproducts should be an ideal situation for a growing cell. However, researchers found that the cells’ best performance occurred when both pathways were in use. Increasing the amount of protein increased this efficiency even more.
With this concept in mind, the researchers developed a model for the energy metabolism of both species and were able to reliably predict metabolic switches within the cells.
Not only does this work help to untangle the complex web of clues around how cells make their energy, but it also proposes a potential new standard for studying these pathways in the future and for making cells work more efficiently.