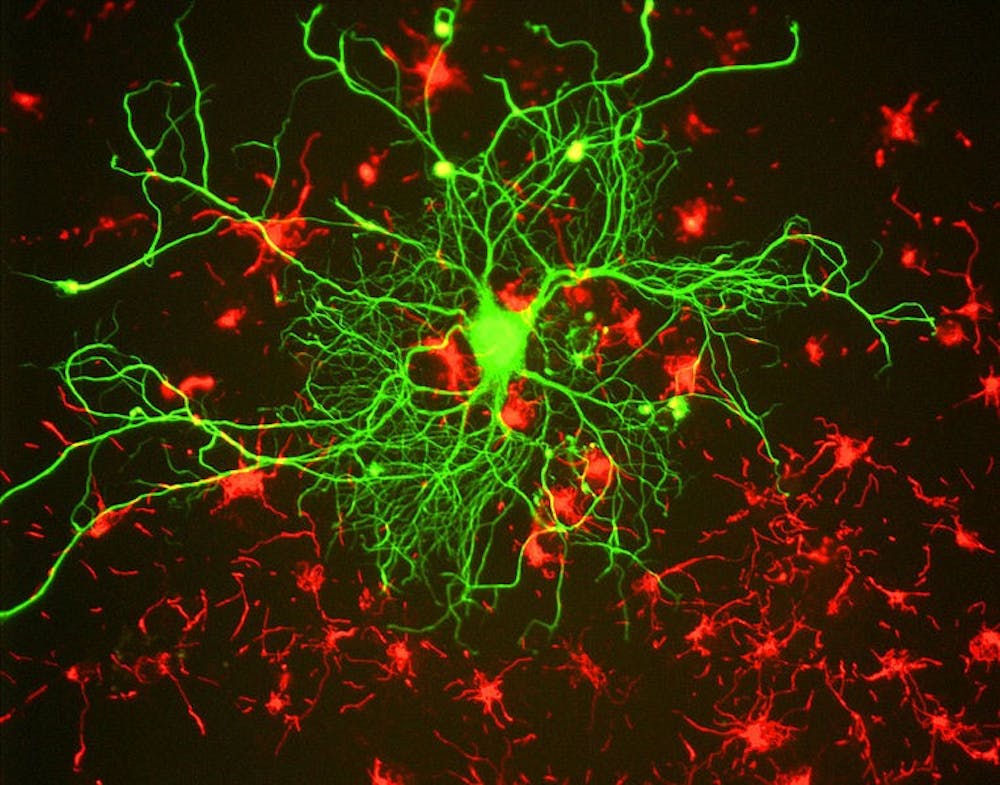
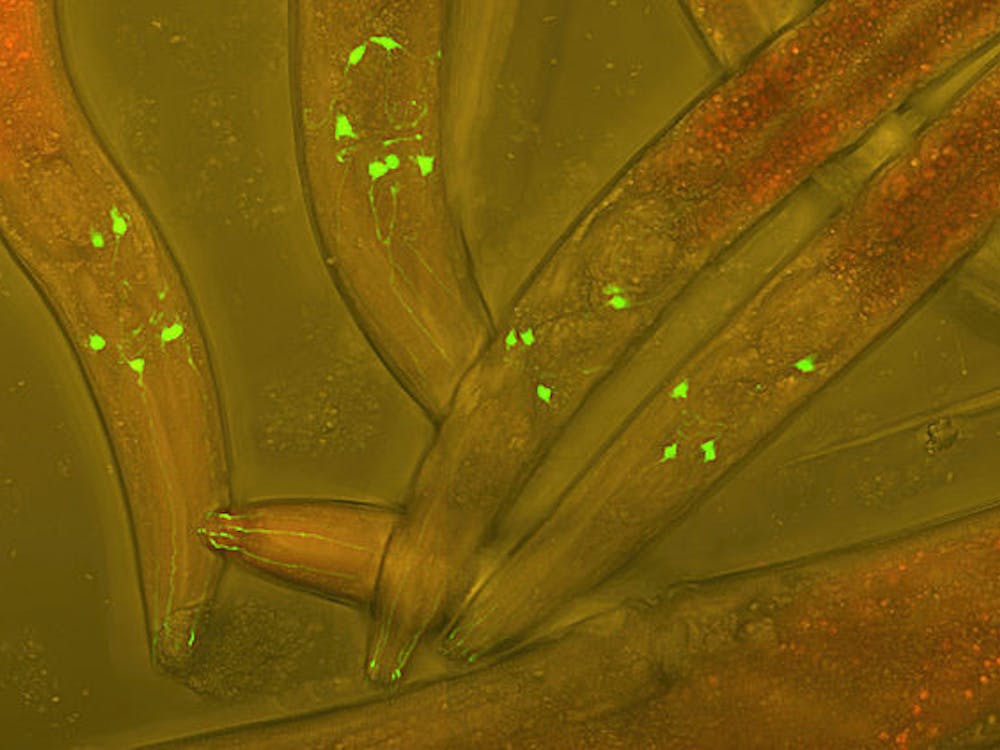
To address this goal, we need tools that enable us to label specific neurons with molecules of interest. Such molecules might allow us to see the entire neuron, how it might connect to other neurons or even change the activity of the neuron to determine its function in a specific behavior.
Recently, an article published in the scientific journal Neuron has statedthat the Karpova Lab at Janelia Research Campus has engineered a new virus tool that can infect axons and travel backwards to the cell body. This opens up new possibilities for understanding neural circuit function.
The adeno-associated virus (AAV) has been a widely important tool for neuroscience. Scientists can package all sorts of genetic material into the AAV, which can then be injected into a specific region in the brain. The AAV will infect the cells of interest and deliver genetic material.
As a result the cell will then express the molecules that we want, such as a fluorescent protein. Moreover AAV is relatively harmless, not toxic to neurons and elicits minimal immune response, making it an ideal vector (or carrier).
Most of the AAVs that are being used in the lab are only capable of anterograde transport. This means that the virus will infect the cell bodies and then spread to the axons. In terms of understanding how neurons connect, this anterograde transport can allow us to determine the output targets of a neuron of interest.
For example, imagine there is neural population A, and we would like to know where population A sends its output to. By infecting population A with an AAV to express a fluorescent molecule and following where the fluorescent-labeled axons go, we can figure out the output targets of population A.
But what if we want to know where population A receives input?
Fundamentally, a neuron receives an input from other populations, modifies that input and then sends output information to others.
Tracing the input target is classically done by using dyes and beads that can go inside the axon endings and travel backwards to neural cell bodies (retrograde transport).
Recently, retrograde tracing can also be accomplished using viral vectors such as the rabies virus, yet this method is still hampered by numerous technical challenges.
Using a massive screen, the Karpova lab was able to discover variants of the AAV that can undergo retrograde transport. Testing the utility of the new virus for systems neuroscience studies, they were able to use the virus to express different fluorescent molecules in input neurons to the basal pontine. The fluorescent molecules allowed them to track retrograde axon tracts and even image neural activity.
How might this new virus help with systems neuroscience research?
As previously discussed, this virus now makes it much easier to trace the pre-synaptic input to a region of interest. This new virus system also might make it more flexible to control neural activity.
Conventionally, if you wanted to manipulate the activity of a specific population, you would directly excite or inhibit activity of that particular region.
However this can be problematic in that this one region could receive inputs from various different regions of the brain, and each of these inputs can relay different pieces of information that lead to different outcomes. With the newly developed retrograde AAV system, it might therefore be easier to manipulate the activity of a region by exciting or silencing the input targets instead.