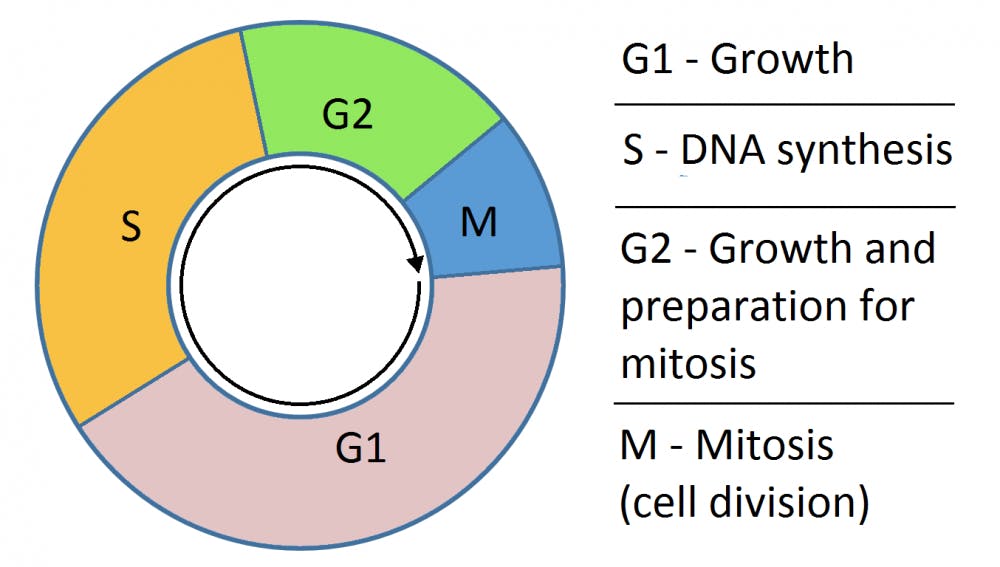
The most widely used and oldest method to analyze adult neurogenesis relies on the delivery of BrdU (5-Bromo-2’-Deoxyuridine), a chemical that permanently integrates into the cell during the S-phase of the cell cycle. This means that, even after cell division, BrdU will be retained in the daughter cells. In the context of neural stem cells, BrdU will be incorporated only in dividing stem cells. After cell division the BrdU-incorporated daughter cells will go on to become neurons.
To confirm that the daughter cells have indeed become newborn neurons, it is necessary to show that they express Neuronal Nuclei (NeuN), which is a gene that is expressed only in mature neurons (other neuronal markers, such as HuC/D, can also be used). As a result, counting the number of cells that have both BrdU and NeuN will yield the number of newborn neurons. The number of newborn neurons that the brain produces is correlated with learning and memory, as well as emotional regulation.
While the BrdU method is useful for obtaining the number of newborn neurons, BrdU is limited to the cell’s nucleus. The BrdU method alone consequently does not yield any further information regarding the labeled cell’s overall morphology or shape, which are crucial for the cell’s function. In order to study the overall morphology of the newborn neuron, such as its axons and dendrites, neuroscientists have turned to retrovirus. Retroviruses are useful in that they not only infect newborn neurons, but also spread throughout the entire cell, allowing for the visualization of axonal and dendritic structures.
Together BrdU and retrovirus are two powerful methods that can be used to analyze the production of newborn neurons and their morphology. However both methods suffer from several important limitations. Whereas BrdU is injected intraperitoneally (into the body cavity), retrovirus is injected directly into the brain. These external deliveries can result in a higher variance in the number of cells infected, making it more difficult to obtain reliable cell counts. The retrovirus itself also has highly stochastic infection properties.
Improving the consistency of the retroviral injection rate will allow the retrovirus to be used not only for determining the morphology of new neurons, but also for performing cell counts, similar to BrdU methods.
Direct injection of retrovirus into the brain also poses another problem, namely inevitable injury at the injection site. Previous studies have shown that mechanical injury can induce cellular proliferation and potentially neurogenesis at the region of injury. In other words, it is possible that some of the neurons infected by the retrovirus might include those that were born in response to the injection-induced injury, making it harder to interpret the results from studies using retrovirus. Improvements to the delivery method of retrovirus would be extremely useful for neurogenic analysis.
One potential modification to the virus could be to alter its structure so that it can pass through the blood brain barrier, allowing for systemic delivery methods (such as by IP injection) that do not induce injury at the region of injection.